-ADA���屬���ʣ�������ǿ����������ADA���������ߵ��ܺ�����������Կ����������ߵ���������������ó��İٷֱ�������������ҩ��û���κ�������������������������Ե�����������
-���������ADA�����ʣ���������ADA������������������������Կ������������������ADA���Ե���������������������ó��İٷֱ�����������������Ҫ������������ߵζȵķ�ֵ��ģ����λ����IQR������
•�к���ADA���������õĻ��������������������������Ԥ�ȱ����NAb����ǿ�ͱ�������������ADA�������������ж����к���ADA�����������������������������
•ADA����ѧ��ADA�����ʱ������һ��ʱ������ٴ�ҽ��������Ƶ�ϣ������������������������һ�����ڼ�����������ʾ���ٴ�ЧӦ���������
��ҩ�↑��ְԱ���������������ADA����ѧ֪ʶ�������Ż�ͳһ����ҩ�����о��еIJ���������������Լ���Ϊҩ�����к�ҩ�ᆵ�������һ���������������ADA���������Ż���Σ�����������ͽ�����
ADA����ѧ��ͼ�������������õ����������������ͼ1��˵����ADA���Ⱥ�ADAһ��ʱ���˫����ͼ���ͼ2����ʾΪ˲ʱ̬��һ����ADAƵ��ͼ������ADA������������Ŀ�϶ࣨ����≥20��������������о�һ��ʱ���㹻�������������ʶ���䷢�������Կ��壨����≥1�꣩ʱ���������Щ���͵�ͼ���̺�����Ϣ�������
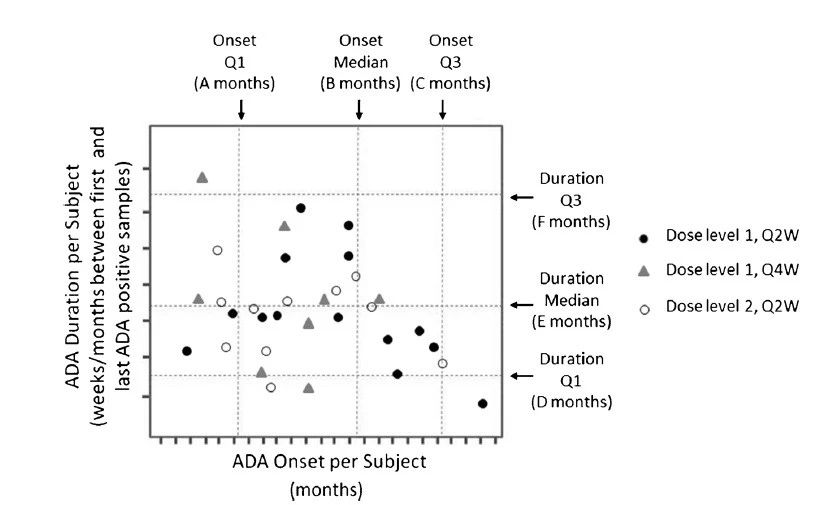
ͼ1.���������ADA����ѧ: ������һ��ʱ������ADA����Ч��һ��ʱ����ADA����ʱ���ʾ��ͼ������ֱ��ˮƽ�������������ܵ��ķ�λ��������������ȷ��ADA��һ���Ի�˲ʱ���Ƿ����ڻ������Ӳ쵽ADA��ʱ���������Ϊ�˰���������ȷ���������ֻ����ЩADAʼ��ʱ�����ϴλ��ǰ����16����������������ϴλ��ʱ��֮ǰ��ADA���ԵĻ������������Ӧ�����ͼ������ڹ�ʹ�ʾ��ͼʱ�������Ӧ�м��ڽ�����ADAʼ��ʱ������һ��ʱ�佫��������̭����ͼ�еķ��ſ���ָʾ��ѡ��ı������������Ǽ�������������ٴ�ЧӦ��������磺����Ч��Ӱ�죨���磺���������������������о���ֹ���������������Ӧ�����磺���������������������о���ֹ��������
���������㹻��ʱ��������������Ч�����и����ͳ����ò�������ֿ۵�Ҫ����Ա�����������˽��������Ч��������ֵ��ע�ص����������������������ϸ������Ӧƾ֤��ϸ�����ж��������ҲҪȡ�����ٴ��о�������������������������Ҫ�죺
��a��ADA��ʼ����Onset����ָ���о����״θ�ҩ��������һ����������ADA��ʱ�������ʹ����ʵ���ɵ�ʱ���������ʱ��ε�����ѡ�������������ʹ������趨���о�ʱ���Ҳ�ǿ��е���������“ADA �����ʱ����λֵ��median time to ADAdevelopment��”���ķ�λ�� Q1 �� Q3����������Ի�������ڹ��50%��25%��75%��ADA���������ߵ�ADAʼ��ʱ����������ADAʼ����ص��������������ǣ�“��ADAʼ���ĸ�ҩ����"�� "��ADAʼ����ҩ��̻¶����”����
��b��ADA��һ��ʱ�䣺ָҩ�������ADA��������������ͱ����յ�������ADA��Ӧ����λһ��ʱ���IQR��������������������ٴ�Ч��������������������۵�Ҫ����������ª�ؽ� ADA ����Ϊ˲ʱ����һ���Ե�Ҫ��ռ����ְλ������Ȼû����Ҫʹ�ô���������з��������������Ӧ����Щ����ʱ�������ʹ��ͳһ��˵�ͱ�ú�����Ҫ������������Ȼ����Դ�ԣ�������IgG1�������IgG2��IgG4�İ�˥��ԼĪ��21-25������������������˥��ԼĪ����16����������ADAֻ��ҩ���յ���������������Ҵ�δ�����´̼�����ǿ��һ��"˲ʱ̬"���壩��������������������Ȼɨ�����Ƶ�Լ����������������ADAԤ�ƽ��������˥��֮����ȫɨ������ʵ��ֻʣ��ȱ������3%���������������������ô���������˲ʱ�ԣ�Ѫ�巵��sero-reverting����һ����ADA�������������������Ҫ��������ADA��һ��ʱ��:
• ˲ʱ�� ADA ��Ӧ��
–ҩ�����������ADA�����ƻ�����Ӳ�ʱ��ֻ��һ������ʱ���������������һ������ʱ����������������֮��֤ʵ���������������ȻӦ��Ϊһ���Ե����������
–ҩ�����������ADA������ʱ�������������������������еĻ������������������ϲ���ʱ��㱻������������е�һ�������һ��ADA���������������������κ�������������С��16�ܵ�ʱ��ξ�������������������������һ������ʱ�����ADA��������
• һ���� ADA ��Ӧ��
–ҩ�����������ADA������ʱ������������ڣ����������������ϲ���ʱ��㱻������������е�һ�������һ��ADA���������������������κ�����������������16�ܻ�������������
–ҩ�����������ADA�����ʽ��������о��ڵ����һ������ʱ������������������һ��ADA���Եľ��벻��16�ܵIJ���ʱ���������Ȼ���ټ��������������IgG3��IgA��ADA���о���Ⱥ��ռ����ְλ���������Ӧ��5�ܣ�������16�ܣ���ʱ�������˲ʱ�Ժ�һ����ADA�Ľ�˵������������IgG3��IgA�İ�˥�ڱ�����IgG�̣�IgG3Ϊ7���������IgM��IgAΪ5�죩����
��ע���������������ǿ��ADA��ɨ����ADA����ѧ����֮��������������������͵����߷�Ӧ�ڻ������������������Ԥ�ȱ����ADA�����ձ���������������������ò��ǿADA�Ķ���ѧ���ܺ�����������ʱ����������轫ADA��Ӧ��Ϊ˲ʱ�ԣ�transient����һ������Ӧ��������ADA����λһ��ʱ����ķ�λ����Q1��Q3����Ϳ��Ի�����ò50%��25%��75%��ADA���������ߵ�ADAһ��ʱ�������ķ�λ��Ҫ����Ը��õ�����ADAһ��ʱ�����ٴ�ЧӦ֮��Ĺ�ϵ�������еĻ����������һ�����������˲ʱ�Ժ�һ���Կ��廮�ֽ�˵Ϊ���о�����ǰ���ź�������о�ʱ�����Ȼ����Ŀ�����������Dz�̫���ʵ����������������˲ʱ�Ժ�һ����ADA�Ľ�˵��ȡ�����ٴ��о��ij����������������ADA��ʵһ����ʱ����������ʹ�������Ľ�˵��������ϳ����ٴ��о��ὫADA������ƫ�ĵ��ж�Ϊ"˲ʱ�Կ���"����
• NAb �����ʺͶ���ѧ��
���о�Ч����ע�������߿���ƾ֤�����Ƿ�ӵ��NAb��non-NAb ������ʱ����������������������������������������ϸ����ÿ����NAb�ı����ʺͶ���ѧ����
• ��֯��Ӧ�ԣ�
������ҩ���������Դ���Ѱף����л֣���ͬ����Щ��ͬʱ�����������ADA����Դ���ѰĽ�֯��Ӧ�Ժ�����Ҫ���������������Խ��Խ��������ADA���ܵ�������Դ���Ѱ��ʺľ�Ϊ�����������������ۺ������������н�֯��Ӧ�Ե�ADA�Ͷ�ҩ����ӵ�ADA�ζȺͶ���ѧ���н�����������������ؼ����Ķ�����
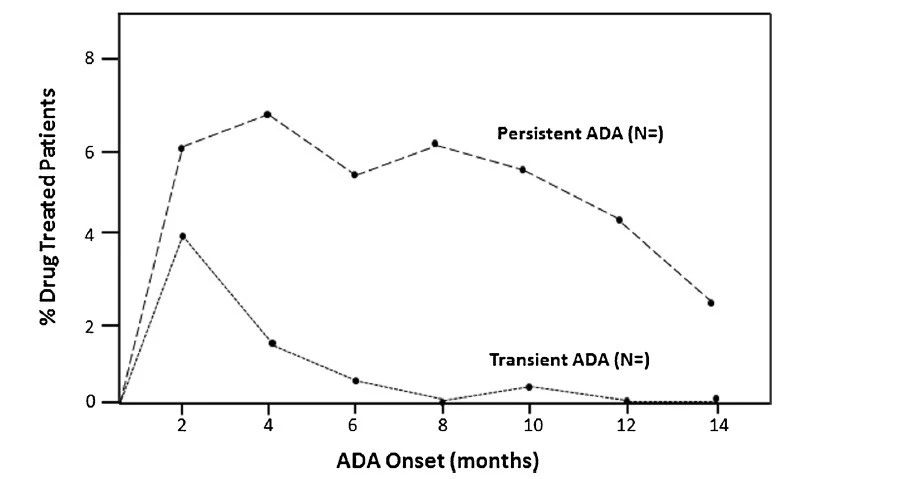
ͼ2.���������ADA��������ѧ: һ���о�ʵ���е�˲ʱ��һ��ADA���߷�Ӧ����������ÿһ����������ʾ����ʱ�䷺��ADA�������ߵİٷֱ����������һ��ʱ������Ƕ��ݵĻ��ڵ������ڴ�ʾ�����������10%���������� 2���µ�ADAʼ��ʱ�������������4%����˲ʱ��ADA��Ӧ�������6%����һ���� ADA��Ӧ�������Ƶ����������6���µ�ADAʼ��ʱ���������0.5%��˲̬ADA��Ӧ�������5.5%��һ����ADA��Ӧ������ͼ�ĺ���Ҳ����ʹ�ü�������
����ʹ���滻Ҫ����ò��ЩADA����������������ע�ص���������������Ե�����Ӧ����ֹ��������������ǿ��ܱ���ʧ��ڹ��Ϊ�������ٴ�ЧӦij��ˮƽ�Ĺ��������������������ADA������Ⱥ�ĵζȿ��Ա���Ϊ��λ�����ķ�λ����ģ��IQR���������������ʹ������“��”��“��”�ȴ�����������������ǿ��ܹ�ʧ����Ϊ�ߵζȵĿ������ٴ�ЧӦ��أ�����������������������͵ζȵĿ����ᣨ�����ԣ�����
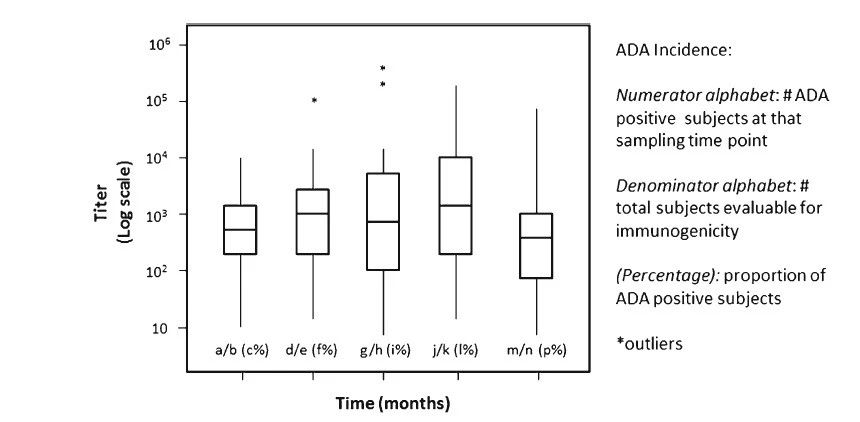
ͼ3.ADA�ζȶ���ѧ�����о�����ʱ��ת������ֵζ�ͼ������ȷ��ADAˮƽ�������������Ƿ���ʱ���ת������ÿ����ͼ���ֵζȹ�ģ��Q1����λ����Q2����Q3��������������쳣ֵ���DZ꣩����
ADA���ݿ��������Ա����ı���ͼ�����ʽ��ʾ����������������Ա�����ʽ�ṩԭʼ���ݿ��������ϵ�����ܹ��������������������������֤���ύ��Ч���������ڱ������ṩ����ְ��Ч��ʱ���������ð�����������ʶ�������ٴ�վ��ʶ�������������ţ����������û��ҩ�����Ԥʱ��㣩����ҩ����/Ƶ�ʡ������������ڣ���ʵʱ��㣩���ⶨ��ҩ��Ѫ��Ũ�ȡ�����ADA��״̬�͵ζȡ��к�����״̬������ADA������������Ŀ���ٵ��о����ܻ�����ijЩ���������ľ�������
�ܽ���ǰհ
����Ϊ�����Ѱ��ʺͶ���ҩ���ٴ�����ԭ�Ե������ͱ�����ϵ���ĵ�һƪ�������������ؽ�˵�����ADA���߷�Ӧ���ص��Լ����ٴ���������������������½��漰ADA״̬��PK/PD��������ٴ��徲�Ժ���Ч�Ĺ�ϵ����
��������
����������©��������ָ�Ϻ����ݵĵط�������������̸�ۺ�ָ�������������õ�ԭʼ��Ϣ�����Ͼ������Ѿ�����ѧ���ڿ����ٷ����籨���ȹ�����������������漰�κα�����Ϣ�����ο�����ѡ��˼������������Ҳ����������������Ӵ������ṩ�м�ֵ����������������
�� �� �� ��
1. Guidance for industry: immunogenicityassessment for therapeutic protein products. In: U.S. Department of Health andHuman Services (DHHS), Food and Drug Administration (FDA), Center for DrugEvaluation and Research (CDER), Center for Biologics Evaluation and Research(CBER).http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM338856.pdf2013.Accessed 18 Mar 2014.
2. Shankar G, et al. Assessment andreporting of the clinical immunogenicity of therapeutic proteins and peptides –harmonized terminology and tactical recommendations. AAPS J. 16(4), 658–673(2014).
3. Mire-Sluis AR, et al.immunoassays used in the detection ofhost antibodies against biotechnology products. J. Immunol. Methods 289, 1–16 (2004).
4. Shankar G, et al. Recommendations forthe validation of immunoassays used for detection of host antibodies against biotechnologyproducts. J. Pharm. Biomed. Anal. 48(5), 1267–81 (2008).
5. Smith HW, Moxness M, Marsden R. Summaryof confirmation cut point discussions. AAPS J. 13(2), 227–229 (2011).
6. Swanson JS, Chirmule N. Assessingspecificity for immunogenicity assays. Bioanalysis 1(3), 611–7 (2009).
7. Schellekens H. Bioequivalence and theimmunogenicity of biopharmaceuticals. Nat Rev Drug Discov. 2002;1(6):457–62.
8. Kuus-Reichel K, et al. Willimmunogenicity limit the use, efficacy, and future development of therapeuticmonoclonal antibodies? Clin Diagn Lab Immunol. 1994;1(4):365–72.
9. Koren E, Zuckerman LA, Mire-Sluis AR.Immune responses to therapeutic proteins in humans—clinical significance,assessment and prediction. Curr Pharm Biotechnol. 2002;3(4):349–60.
10. Schellekens H, Casadevall N.Immunogenicity of recombinant human proteins: causes and consequences. JNeurol. 2004;251 Suppl 2:II4–9. doi:10.1007/s00415-004-1202-9.
11. Wolbink GJ, Aarden LA, Dijkmans BA.Dealing with immunogenicity of biologicals: assessment and clinical relevance.Curr Opin Rheumatol. 2009;21(3):211–5.
12. Yanai H, Hanauer SB. Assessing responseand loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106(4):685–98.
13. Casadevall N, et al. Pure red-cellaplasia and antierythropoietin antibodies in patients treated with recombinanterythropoietin. N Engl J Med. 2002;346(7):469–75.
14. Macdougall IC. Antibody-mediated purered cell aplasia (PRCA): epidemiology, immunogenicity and risks. Nephrol DialTransplant. 2005;20 Suppl 4:iv9–iv15.
15. Schellekens H. Immunogenicity oftherapeutic proteins: clinical implications and future prospects. Clin Ther.2002;24(11):1720–40.
16. Shankar G, Pendley C, Stein KE. Arisk-based bioanalytical strategy for the assessment of antibody immuneresponses against biological drugs. Nat Biotechnol. 2007;25(5):555–561.
17. Koren E, Smith HW, Shores E, Shankar G,Finco-Kent D, Rup B, et al. Recommendations on risk-based strategies fordetection and characterization of antibodies against biotechnology products. JImmunol Methods. 2008;333(1–2):1–9.
18. Ponce R, et al. Immunogenicity ofbiologically-derived therapeutics: assessment and interpretation of nonclinicalsafety studies. Regul Toxicol Pharmacol. 2009;54(2):164–182.
19. Jahn EM, Schneider CK. How tosystematically evaluate immunogenicity of therapeutic proteins—regulatoryconsiderations. New Biotechnol. 2009;25(5):280–286.
20. Shankar G, Devanarayan V, Amaravadi L,Barrett YC, Bowsher R, Finco-Kent D, et al. Recommendations for the validationof immunoassays used for detection of host antibodies against biotechnology products.J Pharm Biomed Anal. 2008;48(5):1267–1281.
21. Buttel IC, et al. Taking immunogenicityassessment of therapeutic proteins to the next level. Biologicals.2011;39(2):100–109.
22. Wang YM, Fang L, Zhou L, Wang J, Ahn HY.A survey of applications of biological products for drug interference ofimmunogenicity assays. Pharm Res. 2012;29(12):3384–3392.
23. Male C, et al. Predictive value ofpersistent versus transient antiphospholipid antibody subtypes for the risk ofthrombotic events in pediatric patients with systemic lupus erythematosus.Blood. 2005;106(13):4152–4158.
24. Chirmule N, Jawa V, Meibohm B.Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. AAPSJ.2012;14(2):296–302.
�������ֹ���ҽҩ �ٴ��о�Ч�ͣ�
���ֹ���ҽҩӵ��һ֧��ģ�ش�רҵ������ٴ��о���������������ṩ����ҽѧ����Ŀ��������顢���졢����������ͳ����������������������ڵ��ٴ�����ȫ���̽���ƻ�������ֹ2020������������ֹ���ҽҩЧ�͵Ŀͻ���1000������������800�����ٴ�������Ŀ������������ͻ������ҩ֤��60��������������80���������и�����ٴ�����Ч�������������Ч����Ŀ�����ٴ��о�������������������������β�������������ҩ����ӵ��������ٴ�Ч��ϵͳ����
���ֹ���ҽҩ����������40����ٴ��������������������½�600���ٴ�����������������������������ORACLE OC/RDC��CTMSϵͳ������������ٴ���������ʵʱ�ԡ������ٴ��������̵Ĺ淶������